Embark on a scientific expedition with our comprehensive freezing point depression lab answers, where the secrets of freezing point depression are unraveled. This in-depth guide provides a captivating overview of the concept, its applications, and the factors that influence it.
Our meticulously crafted answers will empower you to delve into the intricacies of freezing point depression, unlocking its significance in various fields.
Lab Overview: Freezing Point Depression Lab Answers
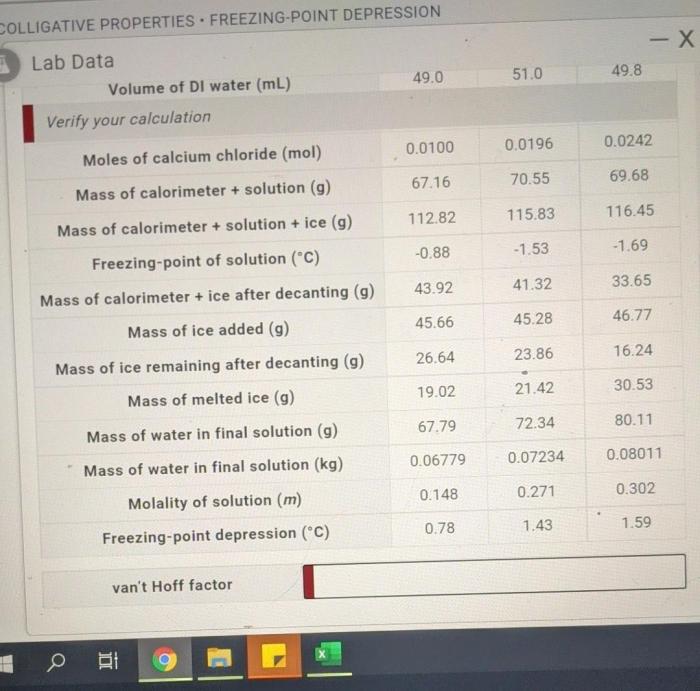
The freezing point depression lab aims to experimentally determine the freezing point depression constant, Kf, for a specific solvent. Freezing point depression is a phenomenon observed when a solute is dissolved in a solvent, causing a decrease in the solvent’s freezing point.
The lab utilizes a variety of materials, including a thermometer, a freezing point apparatus, a solvent (such as water), and a solute (such as sodium chloride). The procedure involves preparing solutions of varying solute concentrations, measuring their freezing points, and analyzing the data to determine the Kf value.
Procedure
- Prepare solutions of varying solute concentrations by dissolving known masses of solute in a known mass of solvent.
- Set up the freezing point apparatus and insert the thermometer into the solution.
- Cool the solution while stirring constantly until it starts to freeze.
- Record the temperature at which freezing begins.
- Repeat steps 2-4 for each solution of varying solute concentration.
Theoretical Background
Freezing point depression is a phenomenon where the freezing point of a liquid is lowered when a solute is added. This is because the solute particles interfere with the formation of ice crystals, making it more difficult for the liquid to freeze.
The freezing point depression is directly proportional to the concentration of the solute. The more solute that is added, the lower the freezing point will be.
Factors Affecting Freezing Point Depression
The freezing point depression of a solution is affected by several factors, including:
- The nature of the solute:Different solutes have different effects on the freezing point of a solvent. For example, ionic solutes tend to cause a greater freezing point depression than non-ionic solutes.
- The concentration of the solute:The more concentrated the solution, the greater the freezing point depression.
- The solvent:The nature of the solvent also affects the freezing point depression. For example, water has a higher freezing point depression than other solvents, such as benzene.
The freezing point depression of a solution can be calculated using the following equation:$$\Delta T_f = K_f
m$$
where:* $\Delta T_f$ is the freezing point depression
- $K_f$ is the cryoscopic constant of the solvent
- $m$ is the molality of the solution
Data Analysis
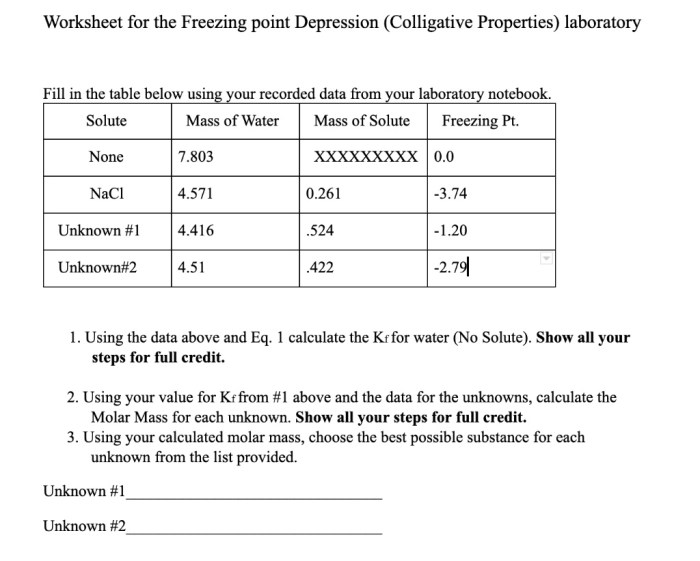
The collected experimental data can be systematically organized in a tabular format, providing a structured overview of the freezing point depression values obtained for varying solute concentrations. The tabular arrangement facilitates the identification of trends and patterns within the data set.
Graphing Freezing Point Depression vs. Solute Concentration
To visualize the relationship between freezing point depression and solute concentration, a graphical representation is constructed. The freezing point depression values are plotted along the y-axis, while the corresponding solute concentrations are plotted along the x-axis. This graph provides a visual representation of the data, allowing for the observation of any linear or non-linear trends.
Calculating and Interpreting the Slope, Freezing point depression lab answers
The slope of the graph obtained from plotting freezing point depression against solute concentration holds significant importance. It represents the change in freezing point depression for a unit change in solute concentration. The slope can be calculated using linear regression analysis.
A steeper slope indicates a greater decrease in freezing point with increasing solute concentration, reflecting a stronger interaction between the solute and the solvent molecules.
Applications
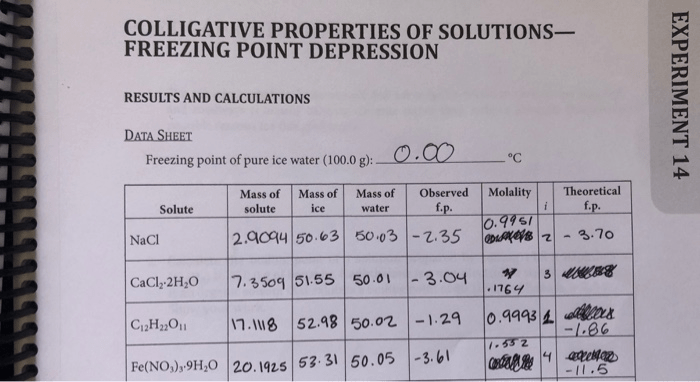
Freezing point depression has various practical applications in different fields.
One notable application is in the determination of the molecular weight of a solute. By measuring the freezing point depression caused by a known mass of solute in a solvent, the molecular weight of the solute can be calculated using the formula:
$$ \Delta T_f = K_f \times m \times i $$
where:
- $$\Delta T_f$$ is the freezing point depression
- $$K_f$$ is the cryoscopic constant of the solvent
- $$m$$ is the molality of the solution
- $$i$$ is the van’t Hoff factor
This method is commonly used in chemistry to determine the molecular weight of unknown substances.
Advantages
- Relatively simple and inexpensive experimental setup.
- Can be used for a wide range of solutes.
- Provides accurate results for non-volatile, non-electrolytes.
Disadvantages
- Not suitable for volatile or highly concentrated solutions.
- Can be affected by impurities in the solvent or solute.
- Requires precise temperature measurements.
Further Research
Ongoing research in freezing point depression focuses on exploring its applications in various fields, including:
- Developing new methods for determining solute concentration and molecular weight.
- Investigating the use of freezing point depression in cryopreservation and food preservation.
- Exploring the potential of freezing point depression for energy storage and thermal management.
Question & Answer Hub
What is the purpose of a freezing point depression lab?
To determine the relationship between freezing point depression and solute concentration, providing insights into solution chemistry.
How does freezing point depression affect real-world applications?
It finds applications in antifreeze formulations, cryopreservation techniques, and determining the purity of substances.
What factors influence freezing point depression?
Solute concentration, solvent properties, and intermolecular interactions play crucial roles in determining the extent of freezing point depression.