Provide a mechanism that explains formation of the following products – Embarking on a journey into the fascinating realm of product formation, this comprehensive guide unveils the intricate mechanisms that govern the creation of diverse substances. Delving into the fundamental principles, chemical reactions, and reaction pathways, we unravel the secrets behind the formation of countless products that shape our world.
From the role of catalysts to the influence of reaction conditions, we explore the factors that orchestrate the transformation of reactants into desired products. By examining the interplay of kinetics and thermodynamics, we gain insights into the dynamics of product formation, enabling us to optimize yields and control product selectivity.
Product Formation Mechanism: Provide A Mechanism That Explains Formation Of The Following Products
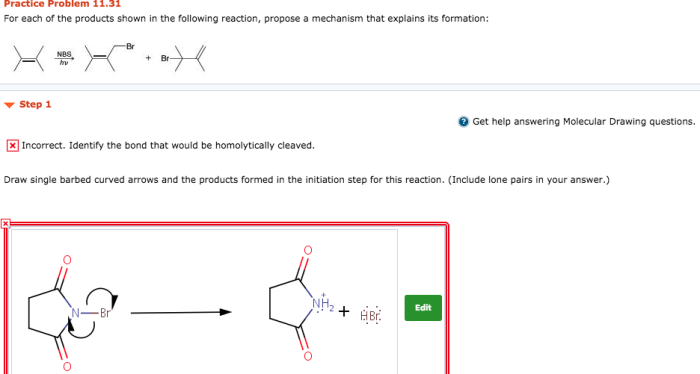
Product formation mechanisms provide a framework for understanding the fundamental principles governing the creation of chemical products. These mechanisms explain the processes through which reactants undergo chemical transformations to yield specific products.
Various product formation mechanisms exist, each with its own unique characteristics. Some common mechanisms include:
- Nucleophilic substitution
- Electrophilic addition
- Free radical reactions
- Pericyclic reactions
The choice of product formation mechanism depends on several factors, including the nature of the reactants, the reaction conditions, and the presence of catalysts.
Role of Catalysts and Reaction Conditions
Catalysts play a crucial role in product formation by providing alternative pathways with lower activation energies. This reduces the energy barrier for the reaction and accelerates the rate of product formation.
Reaction conditions, such as temperature, pressure, and solvent, can also influence the product formation mechanism. These conditions can affect the stability of reactants and products, as well as the rate of reaction.
Chemical Reactions and Product Formation
Chemical reactions involve the transformation of reactants into products through the breaking and formation of chemical bonds.
Types of Chemical Reactions
There are numerous types of chemical reactions, each involving specific mechanisms and bond changes. Some common types include:
- Addition reactions
- Elimination reactions
- Substitution reactions
- Redox reactions
Mechanisms of Chemical Reactions
The mechanisms of chemical reactions involve the following steps:
- Bond breaking: Breaking of existing bonds in the reactants.
- Bond formation: Formation of new bonds between atoms to create products.
- Rearrangement: Rearrangement of atoms within the products.
The specific mechanism depends on the type of reaction and the reactants involved.
Reaction Pathways and Selectivity
Reaction pathways refer to the different sequences of steps that a reaction can take to form products.
Factors Determining Selectivity
The selectivity of a reaction, which refers to the formation of specific products, is influenced by several factors:
- Thermodynamic stability of products
- Kinetic factors (activation energy)
- Presence of catalysts
- Reaction conditions
Examples of Reaction Pathways, Provide a mechanism that explains formation of the following products
For example, the reaction of an alcohol with an acid can proceed through two pathways:
- Nucleophilic substitution (SN2): Direct attack of the nucleophile (alcohol) on the electrophile (acid).
- Elimination (E2): Proton abstraction from the alcohol followed by elimination of a water molecule.
The selectivity of the reaction towards either SN2 or E2 depends on factors such as the structure of the alcohol and the reaction conditions.
Kinetic and Thermodynamic Considerations
Kinetic and thermodynamic considerations play a vital role in product formation.
Kinetics
Kinetics deals with the rates of reactions and the factors that affect them. The rate of a reaction determines how quickly the reactants are converted into products.
Thermodynamics
Thermodynamics deals with the energy changes associated with reactions. The equilibrium constant of a reaction determines the relative amounts of reactants and products at equilibrium.
Influence on Product Formation
Kinetic and thermodynamic factors can influence product formation in the following ways:
- Kinetic control: The formation of products is determined by the rates of the competing reaction pathways.
- Thermodynamic control: The formation of products is determined by the relative stability of the products.
Product Isolation and Characterization

Once a reaction has taken place, the products need to be isolated and characterized.
Isolation Methods
Product isolation methods include:
- Filtration
- Extraction
- Distillation
- Chromatography
Characterization Techniques
Product characterization techniques include:
- Spectroscopy (e.g., IR, NMR, MS)
- Chromatography (e.g., HPLC, GC)
- Elemental analysis
These techniques provide information about the structure, composition, and purity of the products.
FAQs
What are the key factors that influence product formation?
The nature of the reactants, reaction conditions, presence of catalysts, and reaction pathways all play crucial roles in determining the products formed.
How can we control the selectivity of a reaction?
By manipulating reaction conditions, such as temperature, pressure, and catalyst choice, we can influence the reaction pathways and favor the formation of specific products.
What techniques are used to isolate and characterize products?
Methods like distillation, chromatography, and spectroscopy are commonly employed to separate and identify the products formed in a reaction.
